Clay is an amazing material. You can shape it and it holds its form, then you can fire it and it turns into stone. Under the microscope, clay is made up of tiny stacked hexagonal crystals, which are able to slide over each other when lubricated by water. The kaolinite image below is reproduced from the Images of clay archive of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.

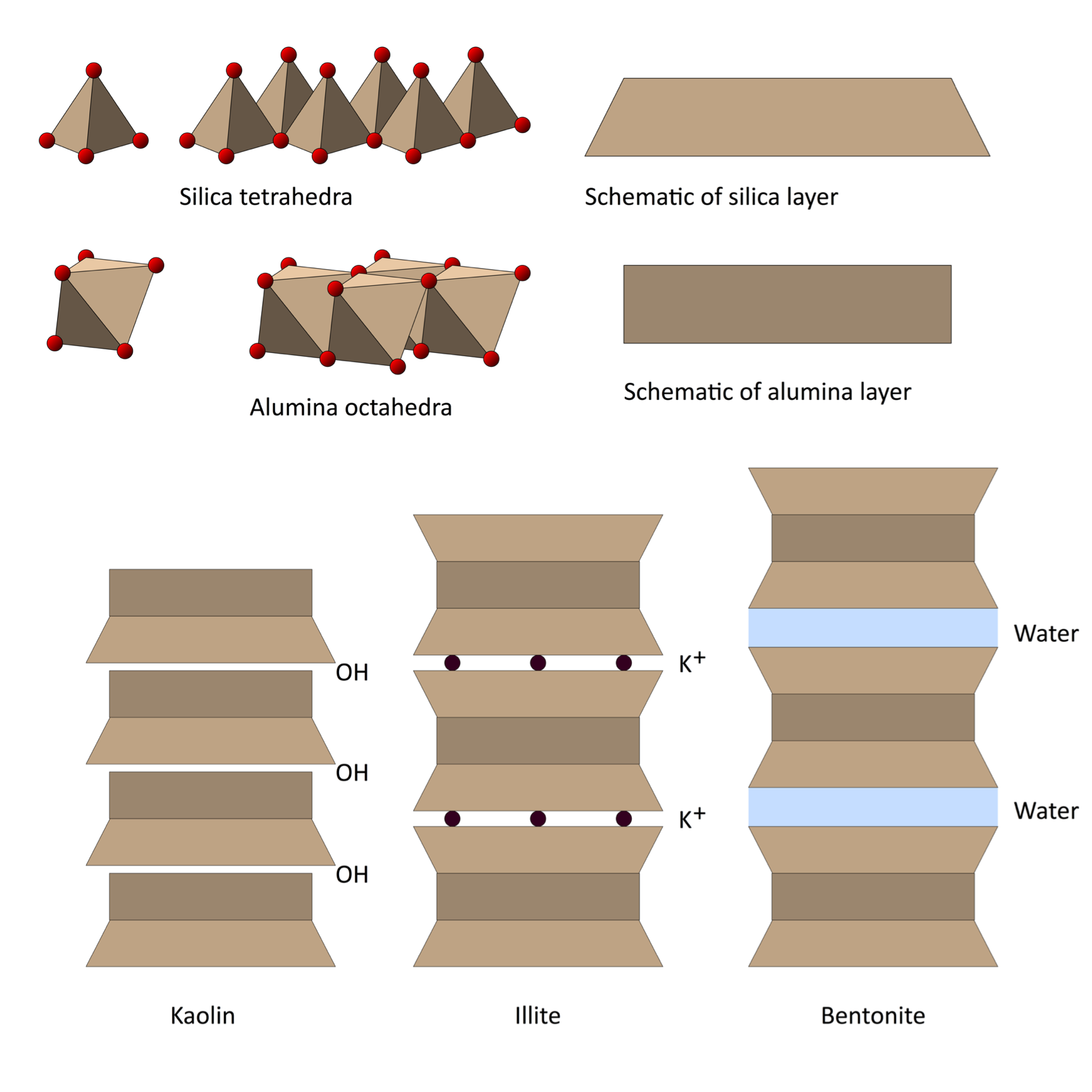
Each clay crystal is made up of thousands of layers of silica tetrahedra and alumina octahedra. These can build up in alternating layers as in kaolin, or in three layers; silica-alumina-silica, as in bentonite. Water can get between the crystals, which enables them to slide easily over each other, allowing the clay to be moulded into shape. Kaolin is less plastic than bentonite as it has a larger particle size. A third type of clay, illite, is derived from mica, and is a constituent of red earthenware clays.
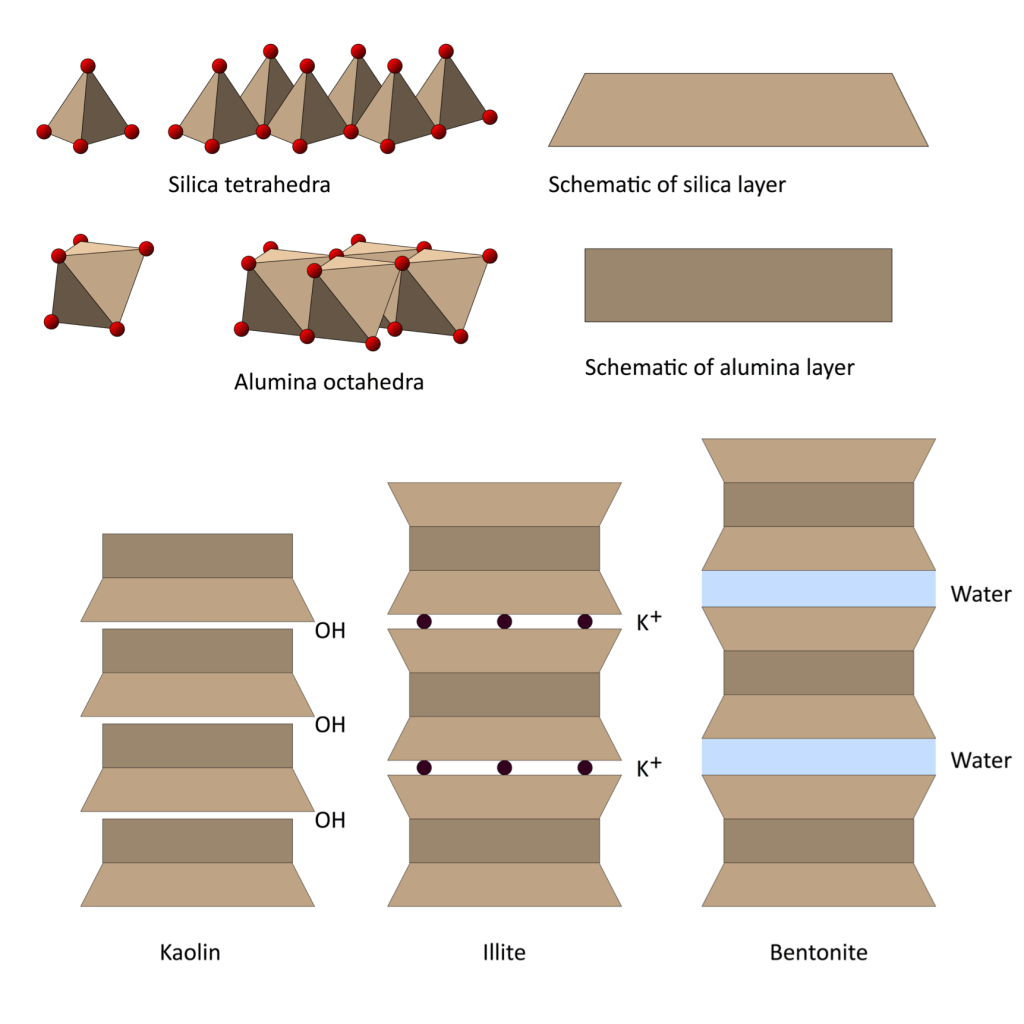
Diagrams by Henry Bloomfield. Read the full article here: Bloomfield_June16
Originally published in June 2016 issue of Ceramics Monthly, pages 64-65. http://www.ceramicsmonthly.org . Copyright, The American Ceramic Society. Reprinted with permission.
